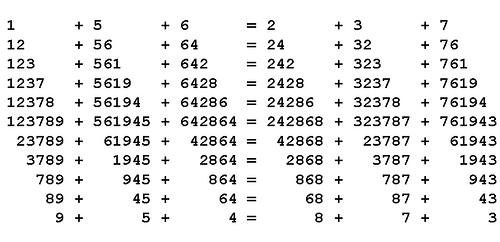In Scripta Mathematica, March 1955, Pedro A. Pisa offers an unkillably valid equation:
123789 + 561945 + 642864 = 242868 + 323787 + 761943
Hack away at its terms, from either end, and it remains true:
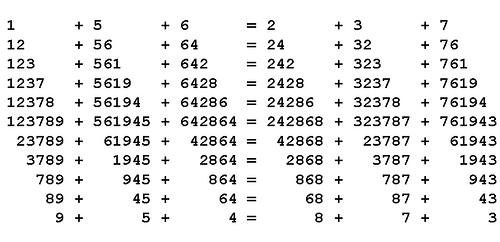
Stab it in the heart, removing the two center digits from each term, and it still balances:
1289 + 5645 + 6464 = 2468 + 3287 + 7643
Do this again and it still balances:
19 + 55 + 64 = 28 + 37 + 73
Most amazing: You can square every term above, in every equation, and they’ll all remain true.
12/03/2016 UPDATE: Reader Jean-Claude Georges discovered that the equalities remain valid when any combination of digits is removed consistently across terms. For example, starting from
123789 + 561945 + 642864 == 242868 + 323787 + 761943,
removing the 1st, 3rd and 5th digit from each number:
x2x7x9 + x6x9x5 + x4x8x4 == x4x8x8 + x2x7x7 + x6x9x3
gives
279 + 695 + 484 = 488 + 277 + 693 (= 1458)
and squaring each term gives
2792 + 6952 + 4842 = 4882 + 2772 + 6932 (=795122).
Amazingly, the same is true for any combination — for example, the equations remain valid when the 1st, 2nd, 4th, and 6th digits of each term are removed. (Thanks, Jean-Claude.)